Somatic Embryogenesis and In Vivo Analysis of
Iron Channels in Sunflower Embryos
X
XuHan, C Brière, N Vallée, C Borin, AAM van Lammeren, G Alibert and
A Souvré
( Cooperation with ENSAT-INP, Toulouse,
France )
|
An experimental research by means of
in vitro morphogenesis, in vivo labeling, cytofluorometry and
confocal laser scanning microscopy
-
An
insight into in vivo ion channel distribution in relation to
sunflower zygotic and somatic embryogenesis.
-
A
systematic cyto-comparison among zygotic embryogenic, somatic
embryogenic, organogenic and other plant tissues.
Main
conclusions obtained:
-
High
homology in PAA labeling among embryogenic protoderm of
zygotic embryo, somatic embryo, and root young epidermis (protoderm),
trichome and root cap, vs epidermis and protoderm in shoot
apex.
-
Callus
and calogenesis devoids of or decrease PAA labeling
respectively.
-
The
above tissues show different PAA labeling patterns, which
indicates a physiologic role played by the tissues in ion
exchange with environment via ion channels.
|
Abstract
A fluorescently labeled phenylalkylamine, DM-Bodipy PAA, was used as a probe for the in vivo detection of ion channels in embryonic and non-embryonic tissues of sunflower. Zygotic embryos, somatic embryos, callus, leaves, roots and shoots were analysed. Fluorescence intensity in the tissues was determined with cytofluorometry and confocal microscopy. DM-Bodipy PAA intensively labeled the protoderm and epidermis cells in both zygotic and somatic embryos. Callus cultures exhibited labeling on sites where somatic embryos developed. Labeling was, however, very weak in leaves, shoots and roots, except in the root cap and in the epidermis of the root. Considering that the location of phenylalkylamine binding sites is related to the distribution of ion channels in both animal and plant cells, the high intensity of labeling observed in the protoderm and epidermis of zygotic and somatic embryos as well as in protoderm, epidermis and caps of root tips, is consistent with the role these tissues may play in ion exchange with the environment.
Key words: Helianthus annuus, embryo, epidermis, protoderm, phenylalkylamine, ion channel
Abbreviation:
DM-Bodipy
PAA =
(5-{3-[3-(4,4-difluoro-5,7-dimethyl-3a,4a-diaza-4-bora-indacen-3-yl)propionamido]phenethyl-N-methylamino}-2-isopropyl-2-(3,4,5-trimethoxyphenyl)-valeronitrile)
(Molecular Probes, USA)
|
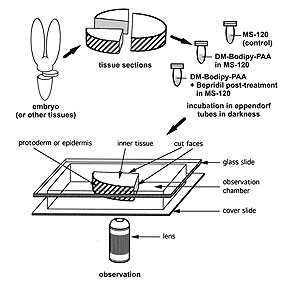
Fig.1: Schematic representation of tissue preparation for Helianthus annuus embryos and probe loading control. The intensity of fluorescence was measured in transverse sections in an observation chamber. Three embryonal regions were analysed: i) the protoderm or epidermis, ii) the inner tissues, and iii) the inner tissues next to the longitudinal/radial cut face that could improve accessibility for the probe.
Figure from Protoplasma
210:52-58, 1999
|
|
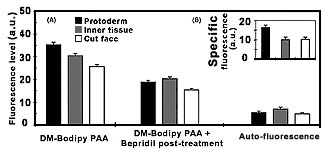
Fig. 2:
Determination of fluorescence intensities of DB-Bodipy PAA labeling in protoderm, inner tissue and cut face of transverse sections of zygotic embryos of Helianthus annuus. (A) Fluorescence intensities were measured after DM-Bodipy PAA labeling (i), after DM-Bodipy PAA labeling plus (-)-Bepridil post-treatment (ii), and the autofluorescence of the tissues was determined (control). (B) Specific labeling by DM-Bodipy PAA in the three tissue regions was deduced by subtraction the values (ii) of the values (i). Data represent the mean
with SE of 80 sections.
Figure from Protoplasma 210:52-58, 1999
|
|
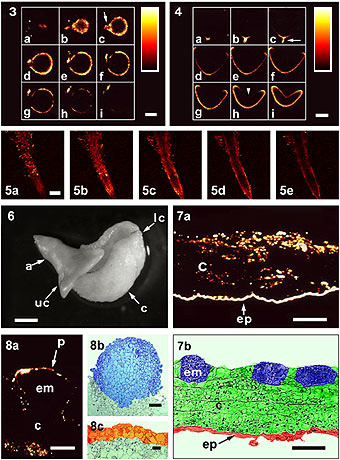
Fig. 3a-i: Series
of optical sections of an intact zygotic embryo of sunflower at the globular
stage labeled with DM-Bodipy PAA and observed by CLSM. Bar = 100 mm.
Fig. 4a-i: Series
of optical sections of an intact zygotic embryo at the heart
stage labeled with DM-Bodipy PAA and observed by CLSM. Bar = 200 mm.
Fig. 5: Series of optical
sections of an intact root tip of a zygotic embryo at the
globular stage labeled with DM-Bodipy PAA and observed by CLSM from outer
region (a & b) towards the median sections (c-e). Bar = 1 mm.
Fig 6: Immature zygotic
embryo in culture for 8 days. The lower cotyledon (lc), which is in contact
with the culture medium, developed callus (c); the embryo axis (a) and the
upper cotyledon (uc) developed normally. Bar = 1mm. Fig.
7: a) Confocal optical section of an epidermal strip in culture labled
by DM-Bodipy PAA. b) Thin section of similar tissue showing embryo
initiation site (em), callus (c) and epidermis (ep). Bar = 100 mm.
Fig. 8: a) Somatic embryo (em) and its
attached callus (c) observed by CLSM after DM-Bodipy PAA labeling. b-c)
Another somatic embryo observed by light microscopy. Bar = 20 mm.
Figure from Protoplasma 210:52-58, 1999
|
|
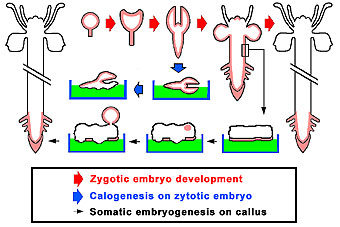
Fig. 9:
Schematic representation of DM-Bodipy PAA labeling (red shadow) in embryos
and plants of sunflower during the development of zygotic embryos to plants
(thick red arrows), during the culture of zygotic embryos (thick blue arrow)
and during the culture of hypocotyl strips giving rise to somatic embryos
(think black arrow).
Refer to Protoplasma 210:52-58, 1999
|
|
References
- Andrejauskas E, Hertel R, Marm?D (1985) Specific binding of the calcium antagonist [3H] verapamil to membrane fractions from plants. J Biol Chem 260 : 5411-5414
- Cho HT, Hong YN (1996) Effect of calcium channel blockers on the IAA-induced cell elongation of sunflower hypocotyl segments. J Plant Physiol 149 : 377-383
- Graziana A, Fosset M, Ranjeva R, Hetherington AM, Lazdunski M (1988) Ca2+ channel inhibitors that bind to plant cell membranes block Ca2+ entry into protoplasts. Biochem 27 : 764-768
- Harvey HJ, Venis MA, Trewavas AJ (1989) Partial purification of a protein from maize (Zea mays) coleoptile membranes binding the Ca2+ -channel antagonist verapamil. Biochem J 257 : 95-100
- Knaus HG, Moshammer T, Kang HC, Haugland RP, Glossmann H (1992) A unique fluorescent phenylalkylamine probe for L-type Ca2+ channels. J Biol Chem 267 : 2179-2189
- Laux T, Jürgens G (1997) Embryogenesis: a new start in life. Plant Cell 9 : 989-1000
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI (1997) Roles of higher plant K+ channels. Plant Physiol 114 : 1141-1149
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15 : 473-497
- Norris DK, Bradford HF (1985) On the specificity of verapamil as a calcium channel-blocker. Biochem Pharmacol 34 : 1953-1956
- Péissier P, Bouchefra O, Péin R, Freyssinet G (1990) Production of isolated somatic embryos from sunflower thin cell layers. Plant Cell Rep 9 : 47-50
- Piñeros M, Tester M (1997) Characterisation of the high-affinity verapamil binding site in a plant plasma membrane Ca2+-selective channel. J Membrane Biol 157 (2): 139-145
- Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113 : 111-118
- Sterk P, Booij H, Schellekens GA, Van Kammen A, De Vries S (1991) Cell-specific expression of the carrot EP2 lipid transfer protein. Plant Cell 3 : 907-921
- Terry BR, Findlay GP, Tyerman SD (1992) Direct effects of Ca2+-channel blockers on plasma membrane cation channels of Amaranthus tricolor protoplasts. J Exp Bot 43 : 1457-1473
- Thomine S, Zimmermann S, van Duijn B, Barbier-Brygoo H, Guern J (1994) Calcium channel antagonists induce direct inhibition of outward rectifying potassium channel in tobacco protoplasts. FEBS Lett 340 : 45-50
- Thuleau P, Graziana A, Canut H, Ranjeva R (1990) A 75-kDa polypeptide, located primarily at the plasma membrane of carrot cell-suspension cultures, is photoaffinity labeled by the calcium channel blocker LU 49888. Proc Natl Acad Sci USA 87 : 10000-10004
- Timmers ACJ, Reiss HD, Boshung J, Traxel K, Schel JHN (1996) Localization of calcium during somatic embryogenesis of carrot (Daucus carota L.). Protoplasma 190 : 107-118
- Vallée N, Brière C, Petitprez M, Souvré A, Alibert G (1997) Studies on ion channel antagonist-binding sites in sunflower protoplasts. FEBS Lett 411 : 115-118
- Vroemen CW, Langeveld S, Mayer U, Ripper G, Jürgens G, Van Kammen A, De Vries SC (1996) Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 8 : 783-791
Ward JM, Pei Z-M, Schroeder JI (1995) Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 7 : 833-844
- XuHan X, Souvr?A, Granier C, Petiprez M (1995) An improved immunolabeling method for microtubular cytoskeleton in poplar free nuclear endosperm. Biotech Histochem 70: 252-257
- XuHan X, Van Lammeren AAM (1997) Structural analysis of embryogenesis and endosperm formation in celery-leafed buttercup (Ranunculus sceleratus L.). Acta Bot Neerl 46 : 291-301
- Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5 : 1371-1381
Return to R&D
IRIT-ARI
Publications
|
|
|
|
|
