MADS genes FBP7
and FBP11 functions during Petunia ovule initiation and early
development
Xiao-Fei Cheng, Peter E. Wittich, Henk Kieft, Gerco
Angenent, Xu XuHan, André A.M. van Lammeren
( EU FP5 project.
Cooperation with PCB, PRI, WU, the Netherlands, and Chinese Academy of Forest
Sciences, China )
|
Abstract
The temporal and spatial distribution of the Petunia Floral Binding Proteins 7 and 11 (FBP7/11) were determined immunocytochemically during to ovule initiation and development. In wild type plants, FBP7/11 were first detected in the placenta before ovule primordia were formed. At ovule primordium stage, FBP7/11 levels increased in the placenta, and appeared in ovule primordia at the sites where integument primordia developed. At megagametogenesis stage, FBP7/11 were present at high levels in placenta, funicle and integument, but not in nucellus and gametophyte.
Transgenics with cosuppression of FBP7/11 formed normal ovule primordia on the placenta from which both normal ovules and carpel-like structures developed. The amount of FBP7/11 was low in the ovules and undetectable in the carpel-like structures. Plants with ectopic expression of FBP7/11 developed normal ovules on the placenta, and, in addition, ovule- and carpel-like structures on sepals. Placental and sepal ovules showed the same labeling pattern as observed in wild type ovules. FBP7/11 levels were, however, low or undetectable in the carpel-like structures.
The results indicate that FBP7/11 only have indirect roles in ovule primordium initiation. At least low quantities are needed for proper ovule differentiation. Thus, the amount of FBP7/11 is related to the type of development after primordium formation i.e. towards the formation of real ovules or carpel-like structures.
Key words: Petunia, ovule development, immunocytochemistry, FBP7/11, MADS-box genes
Abbreviation:
FBP: Floral Binding Protein
|
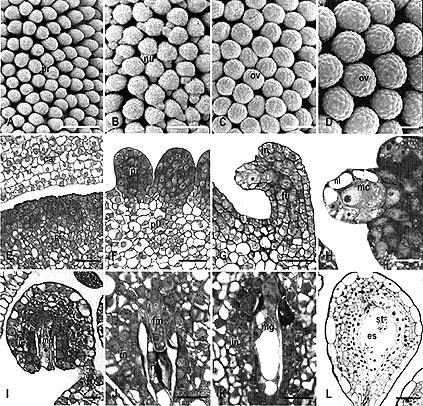
Fig. 1: Ovule development in WT Petunia observed by scanning electron microscopy (A-D) and light microscopy (E-L). Sections were stained with toluidine blue.
A Ovule primordia (pr) on the placenta at stage 1 (bud length 6 mm). B Bent ovules with elongated nucellus (arrow - nl) and initiation of integument at the base of the nucellus at early stage 3 (bud length 9 mm).
C Anatropous ovules (ov) at middle stage 3 (integument shorter than nucellus, bud length 10 mm).
D Anatropous ovules (ov) at stage 6 (bud length 50 mm). E Detail of placenta (pl) before ovule primordium formation (stage 0).
F Detail of ovule primordia (pr) formed on placenta (pl) (stage1). G Overview of a bent ovule with developing megaspore mother cell (arrow) at early stage 3. in integument, fu funicle.
H Detail showing integument (in) formation and the one-cell-layer thick nucellus (nl) covering the megaspore mother cell (mc).
I Anatropous ovule with elongated integument (in) and nucellus surrounding the mature megaspore mother cell (mc) at late stage 3.
J Detail of ovule at stage 5, showing the degenerating nucellus (nl) surrounding the functional megaspore (fm). in integument.
K Detail of ovule with developing megagametophyte (mg) at stage 6. The nucellus degenerates.
L Mature ovule with a cellularized embryo sac (es) at stage 8, showing accumulation of starch grains (st) in the central cell. Bar size for A-D = 100
祄; E-G and L = 40 祄; H-K =20 祄.
|
Figures from Plant Biology 2:693-702, 2000
|
Fig. 2:
Petunia ovule development on the placenta of the FBP7/11 cosuppression
mutant T27017 (A-D) and on the sepals of the FBP11 ectopic expression mutant
T46008 (E-F, H-O) observed with light microscopy (A-D, E, L-O) and scanning
electron microscopy (F-K). A Overview of the placenta with ovules (ov)
after removing the carpel at mature ovule stage. Note that normal ovules are
formed in the middle and basal part of the placenta and that carpel-like
structures developed at the distal part. B Detail of placenta (pl)
with ovule primordia (pr) at stage 1. C An aberrant ovule at mature
stage with a malformed nucellus杔ike structure at the site of the embryo
sac. in integument; nl nucellus; fu funicle. D Overview of a normal
ovule at mature stage, showing a well developed embryo sac with starch
grains. E-O Ovule development on the sepals of the ectopic expression
mutant T46008.
E Detail of the basal part of a flower at mature stage with the petal
tube (p) surrounded by sepals on which ovules (arrows) and carpel-like
structures (cl) are formed. The insert is the overview of the same flower
with one sepal removed. F Detail of the adaxial epidermis at the
fusion area of two sepals at stage 1 (bud length 3 mm), showing a hairy edge
at the left hand side, and three ovule primordia. Note the placenta-like
appearance of the surface of the sepal. G Detail of the adaxial sepal
epidermis of the WT petunia, showing trichomes. H Ovule-like
structures on the sepal at stage 3 (bud length 5 mm), showing bent ovules
with the nucellus only partly covered by the integument. I Developing
anatropous ovules on a sepal at stage 4 (bud length 9 mm). Note that the
nucellus of one ovule is still not covered by the integument (arrow). J
Mature anatropous ovules on sepal at anthesis. K Aberrant primordium
development at stage 3 (bud length 6 mm). The elongated structure lacks
nucellus and integument and will probably develop into a carpel-like
structure. L Light micrograph of section showing an ovule-like
structure at the adaxial side of the sepal. M-O Cleared ovules from sepals
of T46008 flowers at anthesis. M An ovule with normal embryo sac with
egg apparatus (ea). cc central cell, and in integument. N Ovule-like
structure with nucellus-like cells (nl) at the place of the embryo sac. in
integument. O Ovule with an integument (in) that does not cover the
nucellus-like cells (nl). Embryo sac is not present. Bar size for A and E =
1 mm; for B, G and K =50 µm; for C, M-O =25 µm; for D and L = 20
µm for F, H-J =100 µm.
|

Figures from Plant Biology 2:693-702, 2000
|
Fig. 3: Immunolocalization of FBP7/11 proteins on sections of wild type
(A, B, D, G and K) petunia, the FBP7/11 cosuppression mutant T27017 (H-J), and the FBP11 ectopic expression mutant T46008
(C, E, F, L-M). The NBT precipitate (blue signal) is detected by bright field microscopy.
A Overview of WT placenta (pl) before ovule primordium formation, showing clear FBP7/11 signal in the nuclei of the vacuolated cells in the inner zone, and a low level in epidermal
(ep) and subepidermal layers. ca Carpel, B Ovule primordia on WT placenta, showing clear FBP7/11 signal in the placenta (pl) and a low level in ovule primordia (pr).
C Ovule at archespore (ar) stage in T46008, showing high labeling in the placenta (pl) and low labeling in integument primordia (in) and funicle (fu) of the ovule.
D WT ovule at meiosis, showing high labeling in chalaza and integument (in). Signal is absent in the nucellus cells
(nu) and the diade (mc). E Ovule at four-megaspore stage in T46008, showing increase of FBP7/11 signal in integument (in) proceeding towards the
micropyle. Signal is absent in nucellus (nu) and tetrad (1-4). F Ovule at functional megaspore stage in T46008, showing FBP7/11 signal in placenta (pl) and integument (in). It was absent in the functional megaspore (fm), the three degenerating megaspores and the degenerating nucellus cells (asterisk).
G Mature WT ovule, showing FBP7/11 signal in integument cells (in), but not in the embryo sac
(es) and hypostase (hy). H-J Distribution of FBP7/11 signal during ovule development in the cosuppression mutant T27017.
H Detail of placenta before ovule primordium formation in T27017, showing a very low level of FBP7/11 in the nuclei.
I Ovule primordia on placenta in T27017, showing a low level of FBP7/11 signal in both the placenta and
primordia. J Ovule at mature megaspore mother cell stage, showing again low levels of FBP7/11 signal in integument and
funicle. K Ovule primordia (pr) and WT placenta (pl) stained with pre-immune as a control. Showing no FBP7/11 signal in the nuclei.
L-N Distribution of FBP7/11 signal during ovule development on the sepals of the ectopic expression mutant T46008.
L Cross section of young sepal of flower bud of T46008 before the formation of ovule
primordia, showing clear FBP7/11 signal in the nuclei of the adaxial epidermal and sub-epidermal cells (arrows).
M T46008 sepal with FBP7/11 signal in the cell nuclei (arrows) of an ovule-like structure (left hand side), but not in the carpel-like structure (right hand side).
N A sepal ovule of T46008, showing FBP7/11 signal in the nuclei of the integument (in), but not in the central cell (cc) or egg apparatus (ea).
Bar size for A-C, F, G and N =25 µm; for D and E =10 µm; for H-K =20
µm; for L and
M=50 µm.
|

Figures from Plant Biology 2:693-702, 2000
|
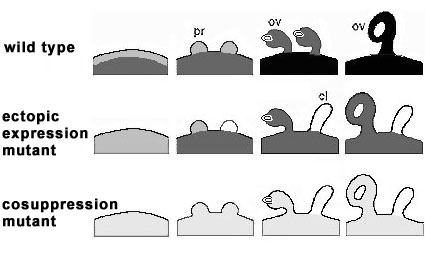
Fig. 4:
Schematic representation of FBP7/11 distribution in the placentae (pl), sepals (se), ovule primordia (pr), ovules
(ov), and carpel-like structures (cl) of wild type petunia (WT), the FBP11 ectopic expression mutant T46008
(EM), and the FPB7/11 cosuppression mutant T27017 (CM). The grey scale mimics the FBP7/11 levels determined by antibody-alkaline phosphatase detection from unlabeled (white) to well labeled (black).
WT FBP7/11 proteins are present in the placenta of WT and EM before ovule primordium initiation, become gradually detectable in the ovule primordia and accumulate in the developing ovules of wild type Petunia.
EM FBP7/11 are present in sepals and developing sepal ovules of EM, but absent in the developing carpel-like structures.
CM During ovule development in T27017 (CM), low levels of FBP7/11 are detected in the placenta, the ovule primordia and developing ovules, but absent in carpel-like structures.
Taking together, it is conclude that initiation of ovule primordia is independent of FBP7/11, low levels of FBP7/11 proteins play a key role in further ovule development and high levels are necessary for proper seed formation. The absence of FBP in primordia results in a lack of specification of petunia ovule identity, and thus to the formation of carpel like structures.
|
Figures from Plant Biology 2:693-702, 2000
|
|
Related References
-
Angenent, C.G., Franken, J., Busscher, M., van Dijken, A., van Went, J.L., Dons, H.J.M., van Tunen, A.J. (1995) A novel class of MADS box genes is involved in ovule development in Petunia. Plant Cell 7: 1569-1582.
-
Angenent, G.C., Busscher, M., Franken, J., Mol, J.N.M., van Tunen, A.J. (1992) Differential expression of two MADS box genes in wild-type and mutant petunia flowers. Plant Cell 4:983-993.
-
Bouman, F. (1984) The ovule. In Embryology of angiosperms, Jori, B.M. ed. (Berlin: Springer-Verlag). 123-157.
-
Ca馻s, L.A., Busscher, M., Angenent, G.C., Beltran, J.P., van Tunen, A.J. (1994) Nuclear localization of the Petunia MADS box protein FBP1. Plant J 6:597-604.
-
Coen, E.S., Meyerowitz, E.M. (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353: 31-37.
-
Colombo, L.C., Franken, J., Koetje, E., van Went, J., Dons, H.J.M., Angenent, G.C., van Tunen, A.J. (1995) The Petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7: 1859-1868.
-
Colombo, L., Franken, J., van der Krol, A.R., Wittich, P.E., Dons, H.J.M., Angenent, G.C. (1997) Downregulation of ovule-specific MADS box genes from Petunia results in maternally controlled defects in seed development. Plant Cell 9: 703-715.
-
Kater, M.M., Colombo, L., Franken, J., Busscher, M., Masiero, S., Campagne, M.M.V., Angenent, G.C. (1998) Multiple AGAMOUS homologs from cucumber and petunia differ in their ability to induce reproductive organ fate. Plant Cell 10: 171-182.
-
Lee, H.S., Chung, Y.Y., Das, C., Karunanandaa, B., Went, J.L. van, Mariani, C., Kao, T.H. (1997) Embryo sac development is affected in Petunia inflata plants transformed with an antisense gene encoding the extracellular domain of receptor kinase PRK1. Sex plant Reprod 10: 341-350.
-
Reiser, L., Fischer, R.L. (1993) The ovule and the
embryo sac. The Plant Cell 5: 1291-1301.
-
Schneitz, K., Balasubramanian, S., Schiefthaler, U.
(1998) Organogenesis in plants: the molecular and genetic control of
ovule development. Trends in Plant Science3: 468-472.
-
Stelly, D.M., Peloquin, S.J., Palmer, R.G., Crane,
C.F. (1984) Mayer抯 hemalum-methyl salicylate: A stain-clearing
technique for observation within whole ovules. Stain Technology
59:155-161.
-
van Went, J.L. (1970) The ultrastructure of the egg
and central cell of Petunia. Acta Bot. Neerl. 19: 313-322.
-
van Went, J.L., Kwee, H.S. (1990) Enzymatic
isolation of living embryo sacs of Petunia. Sex Plant Reprod 3:
257-262.
-
Villanueva, J.C., Broadhvest, J., Hauser, B.A.,
Meister, R.J., Schneitz, K., Gasser, C.S. (1999) INNER NO OUTER
regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes and
Development 13: 3160-3169.
-
Western, T.L., Haughn, G.W. (1999) BELL1 and
AGAMOUS genes promote ovule identity in Arabidopsis thaliana. Plant
Journal 18: 329-336.
Willemse, M.T.M., van Went, J.L. (1984) The female gametophyte. In
Embryology of angiosperms, Johri BM ed ( Berlin: Springer-Verlag).
159-196.
-
Wittich, P.E., de Heer, R.F., Cheng, X.F., Kieft,
H., Colombo, L., Angenent, G.C., van Lammeren, A.A.M. (1999)
Immunolocalization of the Petunia Floral Binding Proteins 7 and 11
during seed development in Petunia hybrida. Protoplasma 208: 224-229.
-
Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.H.,
Feldman, K.A., Meyerowitz, E.M. (1990) Agamous: an Arabidopsis gene
whose product resembles transcription factors. Nature 346: 35-39.
Return
to R&D
IRIT-ARI
Publications
|
|
|
|
